Why is the fireworks so gorgeous?
Fireworks have splendid colors including yellow, orange, green, blue, purple due to the glowing process of metal salts inside it.
How beautiful fireworks are
According to Earth Sky, in chemistry, salt is an ionic compound formed from the reaction between acids and bases. Electrically neutral salt molecules include positive ions and negative ions. For example salt (NaCl) includes positive ions (Na +) and negative ions (Cl-).

Fireworks when burning create many different colors.(Photo: Juan Ramon Rodriguez Sosa / Flickr)
The most common metal used in fireworks is strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks). Purple fireworks often combine a combination of strontium (red) and copper (blue).
Metal salts are inserted into a big piece of a plum inside fireworks called "stars". Launchers and launchers push the fireworks high before exploding. In the process, a slow-burning fuse enters the fireworks core causing it to explode, igniting the star containing metal salts.
The heat from the partial combustion process is absorbed by metal atoms. Electrons surround the low orbit of excited atomic nuclei and jump to higher energy levels. When the heat dissipates, electrons return to a lower energy level or ground state, and release excess energy in the form of light .
Light travels in wave form. Different metal atoms that produce light with different colors are different when stimulated, because the light they produce moves at different wavelengths .
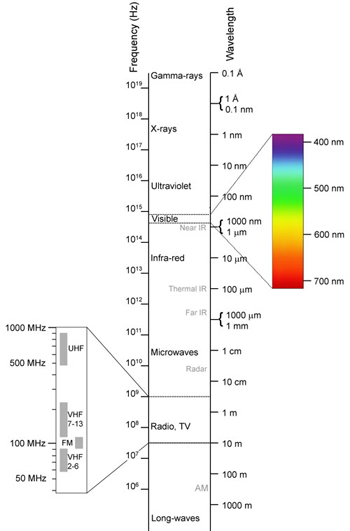
Spectrum of electromagnetic waves.(Photo: Wikimedia Commons)
When metal salts emit light of visible short wavelengths, between 400 and 500 nanometers, they produce purple and blue. Longer wavelengths of light (in the range of 600-700 nm) produce orange and red colors. The average wavelength (500-600 nm) along the electromagnetic spectrum produces yellow and green.
- The birth history of fireworks
- Let's see the structure of each firework
- The incalculable harm of fireworks
- Decode 'secret' fireworks colors
- Making fireworks
- Da Nang glows brightly in the fireworks night
- You probably did not know: Gunpowder was born during the making of elixir
- Farmers invented flood warning column with signal fireworks
- Video: Aircraft drone mounted fireworks release
- Revealing the first fireworks photo in the universe
- Marvel at the fireworks display created by the
- Firework be like?
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? Miracle behind the world's largest stone Buddha statue
Miracle behind the world's largest stone Buddha statue What is alum?
What is alum?