Interesting secrets about oxygen
Inhale . exhale . The 8th element in the Chemistry Periodic Table is a colorless gas, accounting for 21% of Earth's atmosphere. Because it is everywhere, oxygen is easily "disregarded". In fact, oxygen is the most reactive element among non-metallic elements.
>>>Video: What happens if the Earth loses oxygen in 5 seconds?
Practical data on oxygen
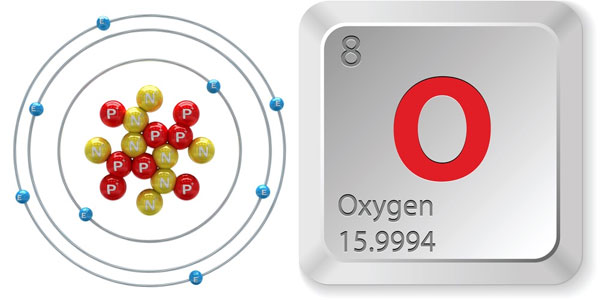
- Number of atoms (number of protons in the nucleus): 8
- Atomic symbol (on the periodic table of chemical elements): O
- Atomic weight (average mass of atoms): 15,9994
- Density: 0.001429 grams in a cubic centimeter
- Status at room temperature: Gas
- Melting point: negative 361.82 degrees F (negative 218.79 degrees C)
- Boiling point: negative 297.31 degrees F (negative 182.95 degrees C)
- Number of isotopes (atoms of the same element with some other neutrons): 11; three stable
- The most common isotope: O-16 (99.757% available in nature)
How is oxygen detected?
According to the US Thomas Jefferson National Accelerator Facility, oxygen is the third most common element in the universe. However, the activity of oxygen makes it quite rare in Earth's atmosphere.
Cyanobacteria are organisms that breathe through photosynthesis, breathe in carbon dioxide and release oxygen, like plants. Cyanobacteria are considered to be the first organisms to produce oxygen on Earth . This is considered a great Oxidation Event in history.
Photosynthesis of cyanobacteria occurs continuously before oxygen appears throughout the Earth's atmosphere. A March 2014 study published in Nature Geoscience found that 2.95 billion year old rocks found in South Africa contain oxides. The rocks were originally in shallow waters, suggesting that oxygen from photosynthesis began to accumulate in the marine environment about half a billion years before it began to accumulate in the atmosphere about 2.5 billion years ago.
Life today depends very much on oxygen, but initially, the accumulation of oxygen in the atmosphere is like a disaster. The new atmosphere causes a series of anaerobic bacteria to become extinct, including those without oxygen. Anaerobic bacteria that cannot adapt or survive when oxygen has been killed in today's new world.
The first vague notions of human existence of oxygen as a chemical element were in 1608, when Dutch inventor Cornelius Drebbel reported that the heating system of Nitrate (potassium nitrate) emitted a gas. The identity of this gas remains a mystery until the 1770s, when there were three chemists almost simultaneously discovering oxygen. Pastor, British chemist Joseph separated oxygen by shining sunlight on mercury oxide and collecting gas from the reaction. He notes that a candle burns brighter when in this gas, thanks to the role of oxygen in the combustion process.
In 1774, the priest announced his discovery. Previously, in 1771, Swiss scientist Carl Wilhelm Steele was the one who actually separated oxygen and had a work on oxygen but did not publish the work. The third person who discovered oxygen was Antoine-Laurent de Lavoisier, a French chemist. He named the element "oxygen" himself. The word "oxygen" and "gene" are all Greek words, meaning "acid-forming".

Do you know?
- When gas, oxygen is not colored. But in liquid form, oxygen is light green.
- If you ever wondered what it would be like to swim in a pool containing liquid oxygen, the answer was: very, very cold. Oxygen only liquefies at a negative temperature of 183 degrees Celsius, so swimming in a liquid oxygen pool will be extremely cold.
- Too little oxygen is dangerous and too much oxygen is dangerous. If we breathe 80% of oxygen for more than 12 hours, we will irritate the respiratory tract, and eventually we can have fluid spills, or edema.
- Oxygen is very heavy. A 2012 study published in Physical Review Letters found that an oxygen molecule (O2) could survive up to 19 million times higher than atmospheric pressure.
- The lowest level of oxygen in human blood was measured near Mount Everest in 2009. Climbers have an average oxygen concentration of 3.28 kilopascal in arteries. Meanwhile, humans normally have an average oxygen concentration of 12-14 kilopascals.
- We have to thank for the right atmosphere with a concentration of 21% oxygen. About 300 million years ago, oxygen levels reached 35%, insects could grow super large: imagine what the world would be like if dragonflies had a wide wingspan of hawks!
Studies of oxygen
In March 2014, physicist Dean Lee of North Carolina State University and colleagues reported that they found the nuclear structure of oxygen-16, the most common isotope of oxygen, in a ground state. (the state in which all electrons are at the lowest possible energy level) and in the first excited state (the next level of energy increase).

The finding is important to understand how nuclei form in stars - from carbon to oxygen to heavier elements. Using simulations on supercomputers and some lattices, researchers were able to see how particles in an oxygen-16 nucleus align themselves. They found that in the basic state of oxygen-16, there were actually four alpha clusters, neatly arranged in a tetrahedral block.
But there is another secret to clarify. The basic state of oxygen-16 and the first excited state have an unusual feature. Both have the same spin - spin is the value that shows how the particles rotate. They also have positive equilibrium, a way to show symmetry.
The simulations gave the answer: In the excited state, oxygen-16 rearranges its nuclei to look like the basic state. Instead of arranging a tetrahedral form, alpha particles reorganize themselves in a square or almost square-like plane. Currently, science is still exploring quantum interactions in oxygen-16 nuclei.
"There are really many interesting things happening inside small things like nuclear," said physicist Dean Lee.
Lee's research aims to understand how oxygen is produced in stars. In addition, there is another form of oxygen research, focusing on the role of oxygen in life on Earth. Immediately after the Great Oxidation event 2.4 billion years ago, oxygen levels may have reached or exceeded today's levels. Many animals have become extinct since then, the simplest animals appeared about 600 million years ago.
Although theoretically, the appearance of oxygen paved the way for the survival of animals, but the story seems much more complicated. Animals did not appear after the first significant collisions of Earth's oxygen levels 2.4 billion years ago. In February 2014, Daniel Mills, a doctoral candidate at Nordic Center for Earth Evolution of Southern Denmark University, together with his colleagues, reported in the PNAS journal that today's modern sponges still exist. can breathe, eat and even grow at oxygen concentrations higher than 0.5% to 4% of the oxygen concentration in today's atmosphere. The sponge is probably the animal that resemble the first animals on Earth.
The finding on sponges reveals an additional aspect of the life of the first animals on earth. Even in modern times, animals such as nematodes also thrive in the oxygen-deprived areas of the ocean.
"Obviously there are many factors that contribute to the evolution of animals, not just oxygen , " Mills said.
- Oxygen appeared early on Earth
- Where does the oxygen for the oxygen mask really come from?
- Video: What happens if the Earth loses oxygen in 5 seconds?
- What happens if we breathe twice as much oxygen now?
- The pilot explained about 10 secrets on the aircraft that were less disclosed
- Scientists have discovered the origin of oxygen in the universe
- The new clue of oxygen isotope originates the solar system
- 10 interesting things about nature you may not know
- 10 interesting psychological facts that help you better understand yourself
- 10 interesting things about cats
- 19 interesting facts about babies make adults suddenly fall back
- Synthesis of substances that can absorb, hold, and release oxygen
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?