First isolated H2O into two types of
That study has just been published in the Nature journal by the Swiss, Chinese and German scientists group.
For the first time, scientists have isolated two different forms of water molecules. Water molecules were previously known to exist in two distinct isomers only slightly different at the atomic level.
Upon successful separation of the two isomers, the team could demonstrate that the isomers react differently in different chemical reactions.
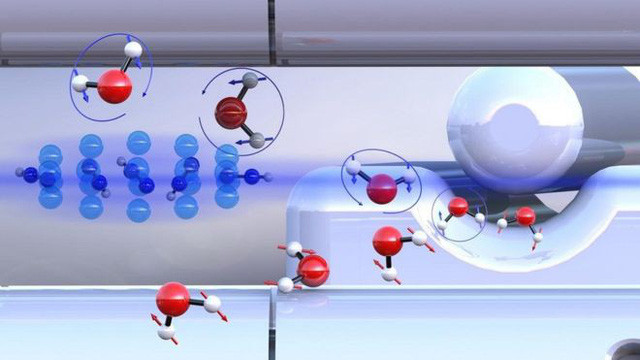
Two different types of water (blue and red arrows) react with diazenylium atoms at different speeds - (Photo: Basel University).
Basically, the water molecule consists of one oxygen atom paired with hydrogen atoms (H 2 O). However, the water molecule can be subdivided depending on the particle properties between the hydrogen bond.
This center is conceived as a spin axis, although it does not actually rotate. In return, the characteristics of this central nucleus affect the rotation of water molecules. If the spins of the pair of hydrogen atoms rotate in the same direction, it will produce ortho-water isomers , if the rotation is different, it will produce the para-water isomer .
These two isomers are "like two drops of water" and it is difficult to isolate them. However, GS. Stefan Willitsch (co-author) and colleagues used electric fields to successfully separate.
To test how two different isomers will react to the substances, the team used ultracold diazenylium ions (a type of nitrogen gas) to test. The team found that para-water reacted 25% faster than ortho-water. Clearly, the rotation state of the H 2 O molecule is affected by spin nuclei , from which the gravitational attraction between the molecules is different.
This conclusion is proved in part by computer simulation.
GS. Willitsch at the University of Basel (Switzerland) said: "The more we have the ability to control molecular states, the easier it is for us to detect fundamental mechanisms and dynamics that cause chemical reactions to occur".
- The 15 most isolated cities on Earth
- Identify stem cells that can heal damage in the heart
- The polygon girl is easily isolated
- The Internet may fall into
- Why do people have different blood types?
- The mystery is hard to explain around the curious neutron star
- Lonely stars
- The land of 120,000 years without the sun in Antarctica
- Rain flood isolated thousands of households in Quang Nam
- The lust of Feng Lan
- The waters were isolated more than 1,500 years at the bottom of the Pacific Ocean
- Secrets of women approaching the world's most dangerous tribe
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?