Real-life atoms are not identical to this figure
This is the symbol that we may have met at least once in our life, possibly in textbooks, places related to 'science' or even in films.

Atomic drawings.
It is often called the "atomic model" but ironically, this is not the real form of the atom and people have known it for more than a century. However, the influence of the symbol is so strong that many people still think that atoms are simply a nucleus and that they revolve around it so beautifully and beautifully.
Where did the first atomic model come from?
To know about the above atomic model symbol, first, let's find out where it comes from. For simplicity, perhaps it should start from the year 1909 (but actually, if you go further, you will arrive until the time of ancient Greece), starting with the discovery of the electron by the English physicist Joseph John Thomson. also called electrons - negatively charged components in atoms. At that time, he proposed that these e were 'captured' within the homogeneous spheres of positively charged matter. It is called this "raisin cake model" because the atoms are in positively charged materials similar to raisins located in pudding.
Later, the New Zealand physicist Ernest Rutherford found that if you fired a positively charged particle into the atoms (in the form of gold foil), not all reflections were in the same way as in a pudding block. ' Large volume. Instead, though still somewhat reflective, most are penetrated, suggesting that electrons must lie around a small mass of a positively charged (nuclear) particle in the middle of space. By 1911, he replicated the discovered model, in which e revolved around a nucleus similar to planets orbiting the Sun. It is also for this reason that this type of atomic model is called an atomic planet model.
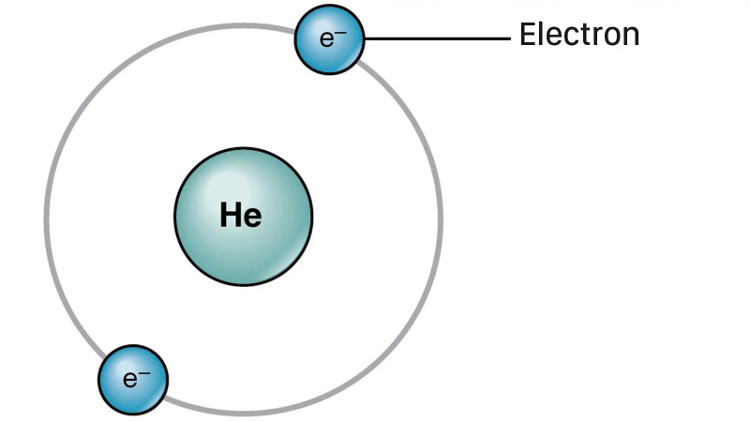
However, if moving in the model of the atomic planet, the e will lose energy due to the orbit around the face, causing them to collide with the nucleus. So two years after Rutherford proposed the model of the atomic planet, Danish physicist Niels Bohr. has solved that weakness: the theory that e does not always follow orbit but instead, they only follow orbit at specific energy levels.
Specifically, e can jump from one energy level to another if they absorb or release energy, but they never deviate from those energy levels. And Bohr's model was also used to illustrate in countless textbooks around the globe (do you still remember the drawing around the nucleus on 2-3 different circles). However, these drawings are also not how the atoms exist in real life.

How was it then?
By the 1920s, physicists discovered that matter also has wave-like properties and atomic orbits are represented by a mathematical function that describes the state as the electromagnetic waves of the e. In a straightforward way, this function is used to calculate the probability of finding e of an atom at any position located in the space surrounding the nucleus.
In other words, the e do not follow certain paths but their appearance in the space around the nucleus is definable. Later, physicists also discovered that they were quantum particles that could exist at different points at the same time. The e still hold independent energy levels but instead of following the path, each e (with presence in many places at the same time) can be visualized by the cloud. And that is why the concept is 'e cloud model'.
It is easy to imagine that it is a space around the nucleus and at any point, the probability of finding e is 90%, that is, every 90 seconds, there are 90 e present at a certain point in the cloud e . In 1932, chemist Robert Mulliken used the concept of 'orbital' to describe the behavior of e in atoms instead of the word 'orbit' to avoid confusion. And finally, it is still impossible to say that Bohr's old model was not good because it was still a simple way, making it easy to imagine a very complex model. And indeed, it is also a very beautiful model that perhaps, anyone who is passionate about science has an indelible impression of it.
- Animals are 100% real but identical
- Observation is released by electrons from atoms
- Image of atom moving in molecule
- Video: Discover the shape of a molecule
- Doraemon's elevated house has appeared in real life in New Zealand
- Atomic memory stores all books in the world in a stamp
- Robot toys are identical to chicks in Japan
- Atoms are 7 times smaller than we have ever known
- Seeing the scowl is seen as a real-life Angry Bird version
- Detecting strange twins
- Discover 2 identical copies of the Milky Way
- Life in the earth comes from Mars
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?