Why doesn't the water freeze all at once and freeze from the top down?
Water is a material that is so familiar to us. It is ubiquitous and an integral part of life. People can live day to day if they are vegetarian or only salty, but anyone can not lack of water to survive.
But perhaps because it is so familiar that people often overlook, not paying attention to this interesting liquid.

Water always freezes from the top down.
What stands out in water is that it often freezes from the top down and then spreads gradually downwards , unlike other substances, to freeze from the bottom up. A clear example is in the rivers and lakes of temperate, tropical areas - where the climate is much colder in the winter, most of which is completely frozen on the surface.
To explain this phenomenon, we need to understand the "quirky" nature of water. When the weather is not too cold or more specifically when the temperature is above 4 degrees C (39.2 degrees Fahrenheit), the water adheres to normal physical rules such as hot and cold.
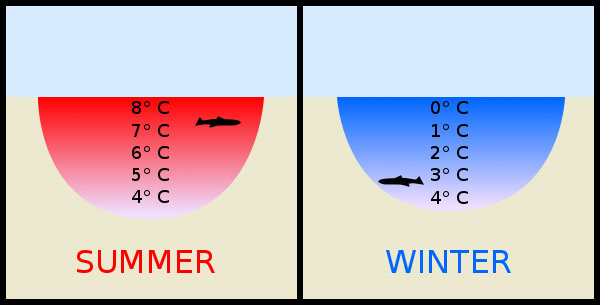
When the temperature is below 4 degrees Celsius, the nature of the water changes 180 degrees, they meet with cold and swell but when there is heat, they contract.
However, when the temperature is below 4 degrees Celsius, things are completely different. At this time, the nature of the water changes 180 degrees, they meet with cold and swell. If the temperature continues to decrease, the density continues to increase and the water (in a solid state) continues to expand. Their mass is inversely proportional to the volume.
That means, with the same amount of water, the solid state of water will be lighter and rise above, the heavier warm water molecules will sink below.Therefore, water always begins to freeze above the previous surface.
In addition, ice is less dense than water due to the way it is made up of a hexagonal crystal structure. Each water molecule consists of two hydrogen atoms linked to the bottom of an oxygen atom. When the ice forms, the hydrogen atoms of one water molecule form a weaker hydrogen bond with the top of the oxygen atoms of the other two water molecules.

In temperate or tropical areas, it is common for the lake to be frozen.
The arrangement of the water molecules in this model takes up more space than if they are randomly shuffled together (as is the case in liquid water). And because the same molecular weight takes up more space when frozen, ice is less dense than liquid water.
"When a lake is cold from above 4 ° C, the surface water loses heat, becomes denser and sinks. This process continues until when all water in the lake is 4 ° C, when the density of water is at its maximum.
If the temperature continues to cool, a more stable, lighter layer of water will appear on the surface. As the layer cools to its freezing point, ice begins to form on the surface of the lake. "
- Does hot water freeze faster than cold water?
- Water can freeze when heated
- Water freeze: new irrigation
- Ice water looks like a tree
- Overwhelmed by the phenomenon of
- Cold to minus 43.5 degrees Celsius in China, boiling water to freeze immediately
- Water freezes right under the surface of Mars
- Beautiful photos: Animals against heat
- New research helps detect life outside the Solar System
- Lasers can freeze water
- Nanotubes can 'freeze' boiling water
- Decipher the mysterious hot water faster than cold water?
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?