Balance chemical reaction with EBAS
For those who are studying the Chemistry program, the balance of chemical reactions has become very familiar. The simple reactions you can easily learn equilibrium but for complex reactions, the memorization of the coefficient will be very difficult to achieve.
Now, with the help of the Equation Balancing and Stoichiometry calculator (EBAS), you can balance any chemical reaction from simple to complex. The program also allows you to calculate the number of moles and the mass of substances in the reaction.
Setting
To install the program, first download EBAS 397KB here. Then, run the downloaded file to start the installation process.
Use the program
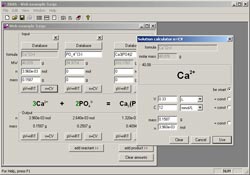 A. Chemical reaction balance : After a successful installation, run the program by double-clicking the test icon of the program on the Desktop. The program interface appears, select File - New to start balancing a new chemical reaction. To be able to easily add more substances in the response, select View - Alternate UI (or press Ctrl + 2 ).
A. Chemical reaction balance : After a successful installation, run the program by double-clicking the test icon of the program on the Desktop. The program interface appears, select File - New to start balancing a new chemical reaction. To be able to easily add more substances in the response, select View - Alternate UI (or press Ctrl + 2 ).
- Enter the chemical formula of the reaction substance in the Substrates section and to add a new substance, click the >> button.
- You do the same as above to enter the chemical formula of the products in the Products section.
- If your formula is correct, after entering the equation, in front of the substances will show the equilibrium coefficient of that substance in the reaction. On the contrary, if the front of the formula shows up the "?" then prove that you entered the wrong. Now you should check again.
B. Some input rules : For common compounds: you enter "_" in front of each indicator. For example, when entering the formula of K 2 SO 4 molecule, you will type K_2SO_4 .
- For the formula of hydrated crystals: you add the "&" character to the water molecule. For example, when entering the formula of CaSO 4 .10H 2 O gypsum you enter as follows CaSO_4 & 10H_2O .
- For ions: you add the front "^" character of the oxidation number and put the oxidation number in 2 {and}. For example, when entering the formula of SO 4 2- you enter the following: SO_4 ^ {2-} .
C. Calculate the number of moles and the mass of the reaction substances : From the chemical equation set above, you can calculate the number of moles as well as the mass of the substances in the reaction.
- Next to each substance in the equation will have the following parts: MW (cubic molecule), n (number of moles), mass (mass).
- Depending on the data of the lesson you enter the value in each cell (you do not have to enter both n and mass because the program will automatically calculate the rest for you). If any substance has no data, you should leave its default value "0". When there is residue in the reaction, the program will automatically redeem you.
- After the data has been entered, the program will show you the results.
The program also adds a function to calculate the number of moles based on the temperature and volume, or the molarity of the substance. Hopefully with the help of this program, you will be more confident in studying chemistry.
Nguyen Huu Khuong
Email : khuonglucky3000@yahoo.com
- Listen to the exciting ASMR sounds of experiments and chemical reactions
- The first photograph of a molecule in a chemical reaction
- Video: Chemical reaction 'The snake of Pharaoh'
- Why is the ability to balance less quickly after age 40?
- Watching beautiful chemical reactions with 4K resolution, you will want to learn Chemistry immediately
- What is fusion reaction?
- Sensor detects explosives in the ground
- Chemical reaction to create more flexible material
- Strange test: Soak your hands in hot bandages
- Video: Decorate beautiful ceramics with chemical reactions
- Recreate the chemical reactions in books through realistic beams
- Have you found a way to 'lock' faster carbon against climate change?
 'Barefoot engineer' invents a pipeless pump
'Barefoot engineer' invents a pipeless pump Process of handling dead pigs due to disease
Process of handling dead pigs due to disease Radiometer
Radiometer Warp Engine: Technology brings us closer to the speed of light
Warp Engine: Technology brings us closer to the speed of light The Artemis space mission comes with a hefty price tag
The Artemis space mission comes with a hefty price tag  America used to spend 22 million USD to study UFOs and this is the result
America used to spend 22 million USD to study UFOs and this is the result  The truth about the author writing the Bible?
The truth about the author writing the Bible?  Do Americans come to the real Moon?
Do Americans come to the real Moon?  NASA called for a contribution to the Asteroid Redirect Mission program
NASA called for a contribution to the Asteroid Redirect Mission program  Mars used to have an ocean
Mars used to have an ocean 