Humans can produce hydrogen and oxygen, so why can't we produce water?
Not only is there all the constituent elements that make up water, we can create this precious resource!
Have you ever wondered . humans have long been able to create oxygen and hydrogen, the two components that make up water, but why don't we produce water to solve the problem of water shortages at Many places in the world?
We all know that water is made up of atoms of hydrogen and oxygen . Two hydrogen atoms linked to an oxygen atom will create a water molecule.

To create water, the first thing we need is hydrogen and oxygen.
This basic chemical theory is no stranger to all of us. But the question is . why don't we apply it to practice to create water.
Everything sounds simple, but it's not as simple as we think.
Theoretically, we can completely create water from the source of hydrogen and oxygen available, but this is an "extremely dangerous" process!
To create water, the first thing we need is hydrogen and oxygen. But not just to bring in two gases to mix well, stir up, we get water. You need to know that, in each molecule of oxygen and hydrogen, electrons, protons and neutrons are strongly linked to each other.
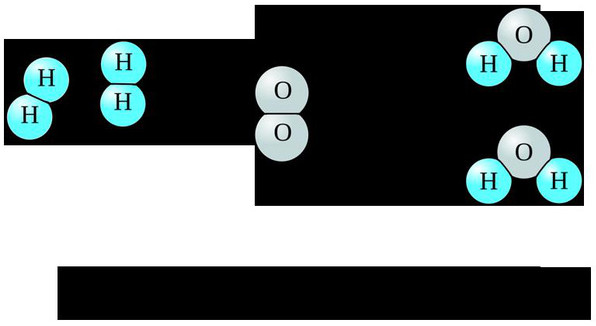
Chemical reactions create water from hydrogen and oxygen.
By bringing these two gases together without any catalytic conditions, the result is just a "air tangle" of two separate hydrogen and oxygen gases.
In order to react, we need to have a "strong explosion" to be able to break down the bonds of oxygen and hydrogen molecules so that their atoms can join together to create water.
Because hydrogen is very flammable and oxygen is a combustion aid, it will not take long to react. What we need is just a spark and a good reaction environment, we will have an explosion to help the reaction take place.
Then the strong bonding of hydrogen and oxygen molecules will be broken, enabling their atoms to combine to create water.
However, this process is extremely dangerous. An explosion reaction on a small experimental scale does not matter. But when conducting experiments on a large scale, enough to create a source of water for the region that is lacking in water, the explosion reaction can take people's lives, even many human lives.
Typically, the Hindenburg balloon disaster occurred on May 6, 1937.
This giant balloon filled with hydrogen gas (a much lighter gas than air, helping a balloon to take off into the air). On May 6, 1937, when it approached New Jersey during a transatlantic voyage, the hydrogen gas chamber caught fire. Along with oxygen in the air that acts as a combustion aid, the hydrogen chamber has exploded and completely burned this giant balloon. Disaster has claimed the lives of 35 people on the balloon.

The Hindenburg balloon disaster occurred on May 6, 1937, killing 35 people.
After the Hindenburd disaster, it was noted that many countries were created. But this doesn't make sense when too many lives have been robbed.
That's why we don't produce water even though there's a plentiful source of hydrogen and oxygen. This process is very dangerous while we have many other options for safer water production.
- The old man monopolized the machine to separate hydrogen from water
- Scientists have demonstrated that it is possible to extract water into oxygen and hydrogen in the universe, while also creating breathing air and fuel for running ships.
- Find an extremely effective, surprisingly cheap hydrogen extraction method
- New opportunities in making hydrogen
- Artificial photosynthesis transforms water into hydrogen and oxygen
- Develop simple, cheap materials for 'artificial photosynthesis'
- Breakthrough in cheap hydrogen fuel production
- How to make new hydrogen
- Effective catalyst to produce oxygen for artificial synthesis
- Water is a catalyst in explosions
- Producing hydrogen from water and sunlight
- The ability to heal the wound of old oxygen
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?