Maybe you don't know: Opening a champagne cap is like launching a rocket
Scientists from France and India used computer simulations to reveal what happens in a few microseconds, when opening a bottle of champagne. They found that in the first millisecond after the cork pops off, the ejected gas forms different shock waveforms - even reaching supersonic speeds - before the foam settles and permeates the substance. liquid.
'We've unraveled the unexpected and stunning flow patterns that lurk just under our noses, every time a bottle of sparkling champagne is opened,' report co-author Gérard Liger-Belair of the University Reims Champagne-Ardenne said, "Who could have imagined the complex phenomena and aesthetics lurking behind such a common situation that any of us have to go through?"

The effervescence of champagne arises from the formation of bubbles on the glass wall of the bottle.
Liger-Belair has studied the physical phenomena of champagne for many years and is the author of Uncorked: The Science of Champagne. He has gained many insights into fundamental physics through opening champagne, just by using laser tomography, infrared imaging, high-speed video photography and mathematical models, among other things. Other methods.
According to Liger-Belair, the effervescence of champagne arises from the formation of bubbles on the glass wall of the bottle. Once they detach from their nucleation site, the bubbles develop as they rise to the surface of the liquid, burst, and deflate. This reaction usually occurs within a few milliseconds, and a distinctive crackling sound is emitted when the bubble bursts. And after the bubbles in champagne burst, they create droplets that release aromatic compounds, which are thought to enhance the flavor of the wine.
In addition, the size of the bubbles also plays an important role in a glass of champagne. The larger bubbles, with a surface size of approximately 1.7 mm, enhance the release of airborne droplets. And they also produce sounds that resonate at specific frequencies, depending on the size. So you can "hear" the size distribution of bubbles as they rise to the surface in a glass of champagne.
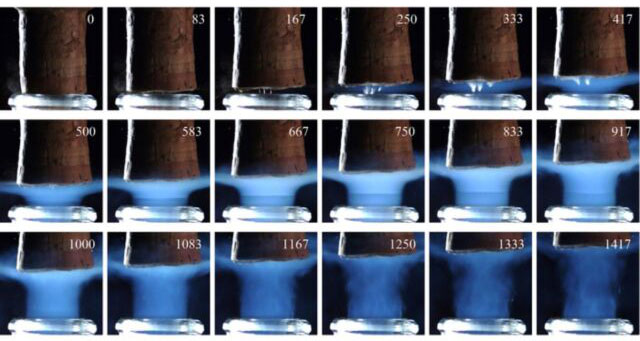
Time series detailing the expulsion of a cork from the neck of a champagne bottle stored at 20 degrees Celsius, images captured through a high-speed camera.
Champagne is often made from grapes picked early in the season, when the fruit has less sugar and higher acidity. The grapes are pressed and sealed in containers for fermentation, just like any other wine. CO2 is produced during fermentation, but it is let out because what one wants at this stage is a base alcohol. Then there is a second fermentation, but this time the CO2 is trapped in the bottle, dissolving into the wine.
It is very important to strike the right balance right now. You need about 6 atmospheres of pressure and 18 grams of sugar, with only 0.3 grams of yeast. If less, the resulting champagne will be too "young", and if it is too much, the pressure will explode the bottle. You also need the right temperature, as this will affect the pressure inside the vessel. That high-pressure CO2 is eventually released when the cork is unscrewed, releasing a steam-laden gas that escapes the bottleneck and into the surrounding air.
Previous tests by Liger-Belair and colleagues used high-speed imaging to demonstrate that shock waves form when a champagne cork is popped. With the present study, "we wanted to better characterize the unexpected phenomenon of gas flow at supersonic velocities, which occurs during the opening of a champagne bottle," said co-author Robert Georges of the University of Rennes 1 said. The typical champagne bottle can be thought of as a miniature laboratory, the researchers say.

And based on those simulations, the team identified three distinct phases. Initially, when the bottle is first opened, the gas mixture is partially blocked by the cork, so the ejection cannot reach the speed of sound. When the cork is released, the airflow can then escape, radiating and reaching supersonic speeds, forming a series of shock waves to equalize its pressure.
These shock waves then combine to form patterns known as shock diamonds (also called thrust diamonds, or Mach diamonds after Ernst Mach, who first described them). . And this is also what is commonly observed in rocket exhausts. Eventually, the jet will slow down to a subsonic rate, when the pressure drops too low to maintain the required pressure ratio between the bottleneck and the cork edge.
According to the scientists, the research has implications for a wide range of applications involving hypersonic flows, including ballistic missiles, wind turbines, underwater vehicles and even rocket launchers.
'The ground below the launch pad as the rocket rises through the air will act as a champagne cork, into which the ejected gases will impact,' the authors said. Thus, the combustible gas ejected from the barrel will create a flow of air at supersonic speed on the bullet. So to speak, the problems are related to the same physical phenomena, and they can be treated with the same similar approach."
- What is surprisingly interesting when it comes to putting raisins in champagne?
- Minh froze the refrigerator does not ruin champagne
- Video: Explain rocket science with 1,000 common English words
- Why can a rocket launch multiple satellites?
- Strange taste of sea champagne
- Helicopter 'captures' the rocket returning to Earth
- Effervescent lake in New Zealand
- A series of memorable opening ceremonies of 7x and 8x generations
- SpaceX missile re-launches set two records in one day
- Blue Origin's rocket after launching still landed and safely escaped
- Humanity is about to witness a rocket hitting the Moon and it is China's 'work'!
- Humans will
 The most famous scientific failures in history
The most famous scientific failures in history Mysterious genius mechanic and the machine froze time
Mysterious genius mechanic and the machine froze time The son carries the 'bad gene' of genius Albert Einstein
The son carries the 'bad gene' of genius Albert Einstein Isaac Newton
Isaac Newton