New discovery of catalysts
Researchers at the University of Virginia, USA, have identified a specific location that triggers the activation of oxygen molecules to produce an oxidation reaction on the surface of the catalyst.
Speaking of catalysts, most people will think of a device to control emissions in exhaust systems of cars to reduce pollution.
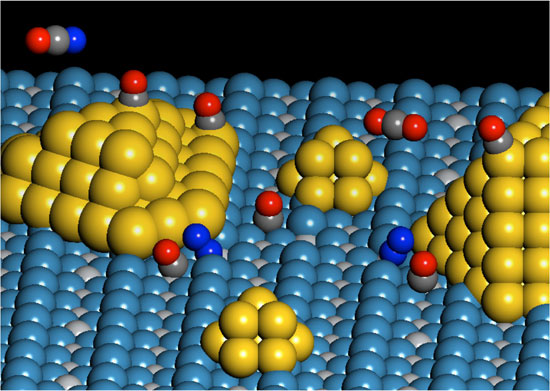
The image of a double catalytic site that catalyzes the activation of an oxygen molecule (dark blue) on the circumference of a gold nanoparticle is held on a titanium dioxide substrate.A carbon dioxide molecule produced by oxidation of absorbed carbon monoxide is released.
But the catalyst is also used for a variety of purposes, including the conversion of oil and gas and renewable resources to fuel, as well as in the production of plastics, fertilizers, paints and solvents. , pharmaceuticals . Approximately 20% of gross domestic product in the United States depends on catalysts to facilitate the necessary chemical reactions to occur, in order to create many products for the daily life
The catalyst is the material that activates the desired chemical reaction without changing itself during the whole process. This allows them to be used continuously because they are not easily damaged and not consumed in these chemical reactions.
For a long time, chemists have discovered and refined many catalysts and continue to do so, although the details of the mechanisms they work on are often not well understood.
During the collaboration of scientific research at the University of Virginia, USA, scientists for the first time determined the specific location of the activation of oxygen molecules to produce an oxidation reaction on the surface. The face of the catalyst, sheds light on the inner workings of the catalytic process. The study was conducted by John Yates, professor of chemistry at the College of Arts and Sciences and Matthew Neurock, professor of chemical engineering at the School of Engineering and Applied Sciences.
The results of the study were published in the journal Science , August 5, 2011.
Yates said that this discovery is of great significance for the understanding of catalysts to take advantage of the potential of many materials, because the oxidation reaction on the surface of the catalyst is very important in some technology applications.
" We have both experimental tools, such as spectrophotometers and theoretical tools, such as computational chemistry, that now allow us to study catalysts at the atomic level, " Yates added. "We can focus on and recognize the specific locations where the catalyst can produce the most efficiency. What we have discovered, will be widely used to create contactants. for all kinds of catalytic reactions. "
By using a titanium dioxide substrate that holds the nanometer-sized gold particles, chemists and chemical engineers found a special place that serves as a catalyst and girth of titanium and particle dioxide substrates. gold. " These positions are special because it involves the bonds of an oxygen molecule with a gold atom and an adjacent titanium atom in titanium dioxide substrate," Yates said. "Gold particles and titanium dioxide substrates." , do not show these catalytic activities when studying them individually. "
By using spectral measurements in conjunction with the theory, Yates and Neurock's team were able to alter specific molecules and determine exactly where this reaction occurred on the surface of the catalysts. .
Experimental and theoretical work, under the guidance of Yates and Neurock, was conducted by Isabel Green, Ph.D. in chemistry, and Wenjie Tang, a researcher in chemical engineering. They demonstrated that important catalytic activity occurred at special locations formed in the belt area between gold particles and titanium dioxide substrate.
" We call this a double catalytic position because it involves two different atoms, " Yates said.
The researchers found that an oxygen molecule chemically binds a gold atom at the edge of a cluster of gold particles and a neighboring Titan atom on a titanium dioxide substrate that reacts with the adsorbed carbon oxide molecule to form. carbon dioxide. By using spectroscopy, researchers can consume carbon dioxide at the double position.
" This is the specific position that causes the activation of oxygen molecules to create an oxidation reaction on the surface of the catalyst ," Yates said.
This study received funding from the US Department of Energy's basic energy research department.
- Discovery of Discovery's
- Discovery ship will fly again
- Discovery is about to take off
- Find a way to turn CO2 into fabric and plastic very quickly
- Solution to use hydrogen instead of fossil fuels
- Comet bombs life bombs on Earth
- Molecular stimulation enhances the performance of energy cells
- Discovery ship boarded the way back
- Do not drink alcohol or beer easily ... die early
- Discovery of Discovery ship may be postponed until February 2011
- Convert CO2 into carbon fuel
- Aircraft assists in reducing air pollution
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?