Why are lithium-ion batteries easy to catch fire and explode?
Under certain conditions, lithium-ion batteries can continuously and uncontrollably heat up, leading to fire and explosion.
According to Lawrence Berkeley National Laboratory (Berkeley Lab) at the University of California, USA, in lithium-ion batteries, the movement of electrons and lithium ions is the main factor that helps generate electricity . However, the process of charging and discharging the battery will often involve a small amount of heat. Under ideal conditions, heat can escape from the battery.

Lithium-ion batteries can experience thermal runaway, leading to the battery overheating, catching fire and exploding (Illustration).
Under certain circumstances, lithium-ions can generate heat many times higher than the rate at which they can escape. This causes the battery to heat up, and can lead to a chain reaction, known as "thermal runaway".
When this phenomenon occurs, the lithium-ion cells inside the battery enter a state of continuous, uncontrollable heating . This is the main reason why the battery becomes too hot, leading to fire and explosion .
Not only that, researchers at Berkeley Lab also pointed out that the presence of local currents inside the battery at rest after fast charging could also be one of the reasons behind the drain phenomenon. heat.
Not stopping there, the design of the batteries also plays a decisive role in the ability to ensure safety against incidents leading to fire and explosion.
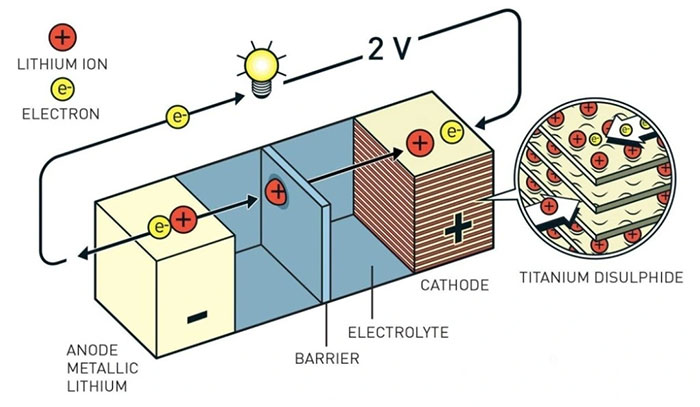
Structure of a lithium-ion battery (Photo: Science Daily).
Normally, because it needs to fit in a compact space, the main components of the battery are often designed to be as small and light as possible. However, this also causes the battery's components to have thin walls, which are quite fragile and have poor impact resistance.
If the battery is punctured or deformed due to physical impact, sparks may occur and cause a fire. This situation can be found in batteries that are poorly processed, unfinished, and of unclear origin.
In a sharing with reporters, Professor. Stanley Whittingham, known as the father of lithium-ion batteries, once specifically warned users about the harmful effects of using poor quality batteries.
He said the safety issue when using lithium-ion batteries stems from the fact that many types of batteries are produced at low cost, so safety is not high, which can lead to fire and explosion during use. use.
GS. Whittingham said to ensure safety, we must make sure our batteries have safety certifications, by buying them at reputable addresses.
Additionally, experts advise that, for businesses that handle a large number of products containing lithium-ion batteries, it is important to understand the risks and how to prevent fires from breaking out.
One of them is to make sure that the place where you store the battery is not exposed to direct sunlight, near objects that emit large amounts of heat, and away from flammable and heat-generating items such as blankets, pillows, and sheets. , cushion.
- Scientists have successfully developed a lithium battery that will never explode despite overheating
- Australia makes batteries that 'like' heat and never explode
- Why does US aviation forbid to carry spare batteries in checked baggage?
- Has found a solution to 'freeze' lithium ion batteries with liquid nitrogen, avoiding batteries to explode if there is a collision
- Air Batteries
- Uses lithium-ion battery detection sensor
- Developed a new lithium-air battery with five times more efficiency than current
- Female Vietnamese graduate student invented lithium battery for life
- Discarded, dissolved in water for 30 minutes
- New lithium battery safer
- Breakthroughs help lithium-ion batteries last longer
- New materials in batteries help to prevent fire
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?