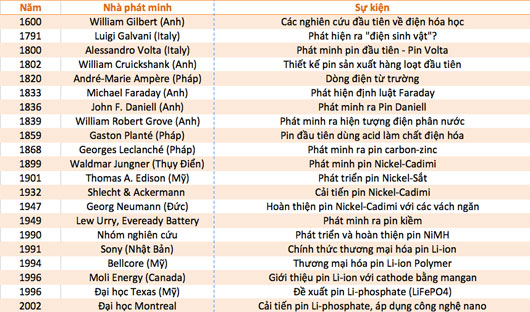
400 year history of battery formation and development
Batteries are a common energy source for many personal and home appliances to industrial applications. There are different types and sizes of batteries that correspond to a lot of devices that consume electricity from wrist watches, children's toys, mobile phones, tablets to large batteries for electric vehicles, . The battery is, is and will be a popular energy storage tool not only in the present but also in the future.
Let's explore the questions surrounding the familiar and important type of device above: When was the first battery made? Who invented the battery? When has the battery been charged? .
Summary of milestones is closely related to battery development
When was the battery invented? 400 years or more than 2000 years ago?
One of the most remarkable and remarkable inventions of humans in the last 400 years is electricity. The first electric currents could have been created before, but it was not until the late 1800s that humanity witnessed specific applications of electricity. It was 250,000 filament bulbs lighting consumer exhibitions in Chicago, USA in 1893 or making a bridge over the Seine, Paris glowing at the World's Fair in 1900.
However, the first electric currents were created by humans many years earlier. In 1963, during the construction of a railway near Baghdad, workers discovered the "Parthian batteries" dating back to 2000 years in an ancient crypt. These are the earliest batteries in human history due to the hands of the Parthians, a people of Northern Persia.
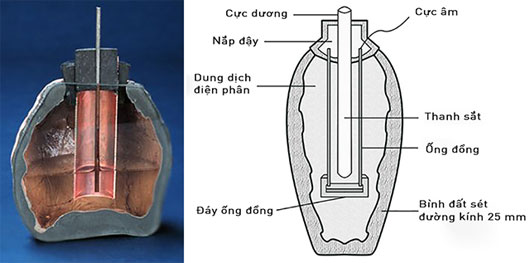
Among the relics found in the tomb, archaeologists found a vermicelli or clay pot filled with vine with a central iron plug and sealed the glass mouth. Around the iron bar is surrounded by tubes wrapped with copper plates. Each vase has a height of about 15cm, copper pipe is about cm in diameter and 12cm long. After reconstructing and experimenting with a similar version, scientists found that the "battery pack" was capable of generating an electrical current of between 1.5 and 2V between iron pillars and copper plates.
Thereby, scientists have predicted that the ancient Parthians used tools to generate electricity to plating gold and silver into objects from the 250s BC. Many scientists argue that the Parthians only use these tools for the purpose of plating, but not as an energy source. Many other archaeological evidence shows that the ancient Egyptians also knew antimony plating on copper items from more than 4300 years ago. Other archaeological sites also show that the Babylonians also discovered and used grape juice technology as an electrolyte to gold-plated jewelry.
1786 - The pair of fake legs died but moved

Professor of the Body of Learning Luigi Galvani (1737-1798) discovered that he stabbed an iron rod into a fake leg placed on a metal table, causing the clones to twitch
In 1786, while conducting a lecture, Professor of the Body of Learning Luigi Galvani (1737-1798) at the University of Bologne, Italy, used a metal rod to stab a skinned frog. Because the frog's body is placed on a metal tabletop, the frog's legs have a twitch. Galvani was surprised by the phenomenon and after a few days of searching, he realized that his legs were twitching when the tip of the metal rod hit and reached the metal table below.
Another day, Galvani used a brass hook to expose his fake legs above an iron bar on the balcony. Galvani had noticed that when the wind blew, the fake legs swung and touched the iron bar, and immediately, the clones would twitch. He pondered to try to explain this strange phenomenon and an idea flashed in his mind: electricity . Galvani concludes that electricity is present in all things and is included in fake feet. He named this type of electricity "electricity of the organism" and published his findings in an article that shocked European scientists with this new type of electricity.
Today, we all know that Galvani mistakenly thought it was the electricity of the creature and he stopped at the phenomenon without understanding the cause of electricity. However, Galvani's discovery came close to the principles that paved the way for making batteries later.
"Volta battery" - The first battery of humanity was born in 1800

Alessandro Volta (1745-1827) was a professor of physics at Pavie University, Italy, the father of batteries
Alessandro Volta (1745-1827) was a professor of physics at Pavie University, Italy. Previously, Volta had many studies to enhance the electrical properties of the bottle of Laiden. Earlier, he proposed a "electric pistol" to make long-distance communications. "Electric Lucid Gun" is connected to an iron wire placed on wooden piles stretching from Milan to Como, Italy. The end of the iron wire is connected to a bottle filled with methane gas. When you want to send an encrypted message, the "electric pistol" will "fire" an electric spark and the receiver will "read" the messages on the methane bottle. However, his model was not really built.
Since Galvani disseminated his findings on "the electricity of creatures" in 1791, in many large European laboratories, a number of scientists have conducted experiments with Galvani's fake legs. Some people connected fake legs with the Leiden bottle (the first form of the capacitor, a glass bottle storing static electricity between the two electrodes inside and outside the bottle) and found that the fake legs had a seizure. bouncing. With this experiment, scientists began to doubt Galvani's "electrophysical" hypothesis. Among the hypothetical protesters was Alessandro Volta.
For the pinch experiment, Volta did not care about the phenomenon of twitching, more profoundly, he tried to find out where the electric power was born to make the clones leg be convulsing. Volta found that the clones were only twitching when there was contact of two different metals. After further research, Volta discovered that electricity generated by chemical reactions and twitching of frog legs only occurs when two different metals are exposed in a salt solution. Specifically, the salt solution exists inside the muscle of the clones.

Volta battery model
Continuing research, Volta carried out a series of experiments using zinc, lead, tin and iron as negative charge sheets (cathode); and copper, silver, gold, graphite as a positively charged plate (anode). After that, he placed polar plates alternately together, separated by a piece of impregnated sponge paper with salt solution. Finally, he connected the first point to the end with a wire and found that a current was flowing. This is the first human battery named "Volta battery" . The reason for the word pin or more accurately the pile set for this device is because this is a stack of round copper and zinc pieces shaped like a pile.

Volta's first battery model is still preserved today
Also in 1800, Volta published his discovery of a stable power supply to the Royal Scientific Council in London in the presence and admiration of many scientists from all over Europe. With this invention, Volta's name is everywhere and is recognized as a person who has contributed greatly to the development of humanity.

The image of Volta is experimenting with the direct observation of the French emperor Napoleon Bonaparte
However, France was the first country to recognize the invention of Volta because during that time, France was trying to approach many advances in science and technology, so it was ready to receive any new ideas. proposed. Soon after, Volta was invited to France and taught at the French Academy of Sciences for his electrical studies. Even in many of his lectures there is the observation of Napoleon Bonaparte.

Cornwall physicist and chemist, UK, Humphry Davy (1778-1829) and battery model
In the same 1800, Cornwall physicist and chemist, UK, Humphry Davy (1778-1829) began experimenting with the chemical effects of electric currents and discovered that electric current was capable of separating The substance in the solution that we know today is electrolysis . Based on Volta's model, Davy created the largest and most powerful electric battery by the time of that time in the basement of the British Royal Academy of Sciences. Witnesses have reported that his battery model has made a brilliant electric arc light chat. In addition, Davy is also famous for detecting N2 O laughing gas or safety mine lamps.

British chemist William Cruickshank with the first battery model design can be produced on an industrial scale
2 years later, in 1802, British chemist William Cruickshank designed the first battery model that could be produced on an industrial scale. Cruickshank proposed a method of using zinc and copper plates of the same size, interspersed with each other, placed in a long rectangular wooden box and fastened. Inside the box there are grooves to hold the metal plates firmly and contain water filled with diluted saline or acid to make electrolytes. This design has advantages over the original Volta model that is not dry and can provide more power. Cruickshank's battery model is like a wet battery that we still use today.
From rechargeable batteries, wet batteries to dry batteries

Rechargeable wet battery from French physicist Gaston Planté
In 1836, the British chemist, John F. Daniell developed a more complete battery version with improved performance and created a more stable current than the original Volta or Cruickshank. However, up to that point, all were primary batteries , which meant that they could only be used once and could not be recharged. By 1859, French physicist Gaston Planté invented the first rechargeable battery . It is a battery with lead plates separated by flannel cloth and placed in dilute sulfuric acid. The battery will be recharged by adding more acid to reuse. This model is still used today under the name of wet battery or wet battery (wet battery ) or carbon zinc battery .

French engineer Georges Leclanché (1839-1882) and his battery model
In 1866 in France, engineer Georges Leclanché (1839-1882) built a wet battery with electrodes immersed in the electrolyte solution. However, shortly after, he took the initiative to improve the battery by using a solution of ammonium chloride and then sealing the battery. This initiative marks the birth of a dry battery generation. The new battery generation allows the battery to be used in a variety of locations, withstand strong vibrations without fear of the electrolyte overflowing like wet batteries. In addition, the battery is made into an inner tube or box containing other parts of the battery such as zinc anodes and cathodes including manganese dioxide and carbon at a ratio of 8: 1 (cathode). ). Electrodes can also contain zinc chloride.
In 1881, Camille Faure built batteries using lead oxide strips as electrodes to replace previous wet battery lead plates. This allows for a much stronger and more stable current. This is the basis for the development of later wet batteries with different types of electrodes.

NiCd battery
By 1899, Swedish scientist Waldemar Jungner invented a nickel-cadmium battery (NiCd) . This is a battery generation that uses nickel as cathode (cathode) and cadmium as anode (anode). However, due to the high cost of manufacturing, NiCd batteries are not widely applied to many users. Two years later, the famous inventor Thomas Edison developed another battery model by using iron to replace Cadimi as an anode to reduce the cost of battery manufacturing materials. Even so, Edison's Nickel-Iron battery model encountered serious disadvantages: low energy, poor performance at low temperatures and high self-discharge capacity. All of the drawbacks on Edison's batteries were not put into production and widely used.
It was not until 1932, Shlecht and Ackermann succeeded in improving NiCd batteries with strong current and long life. The innovative solution of the two inventors is to add the electrode walls to many compartments. In 1947, George Neumann continued to improve the model through the manufacture of a generation of NiCd batteries with many internal walls sealed.

NiMH batteries are familiar to all of us today
Years later, NiCd batteries continue to be the only rechargeable and portable batteries. In the 1990s, environmental issues were of prime interest in Europe and scientists began to pay attention to NiCd batteries due to their ability to handle toxic chemicals after use. The laws were issued to limit the use of these elements and to switch to using more environmentally friendly Nickel-Iron Hydride batteries (NiMH) . However, similar to NiCd batteries, NiMH batteries have not really achieved the desired effect and researchers continue to develop a better battery generation. This is the stepping stone for the creation of lithium-ion batteries (Li-ion).
Li-ion batteries were born and developed to this day

American chemist Michael Stanley Whittingham, the first to propose the idea of a Li-ion battery
The first Li-ion battery was proposed in the 1970s by American chemist Michael Stanley Whittingham (1941) from Binghamton University using titanium sulfide and pure lithium metal as electrodes. However, because lithium is a strong active metal, when exposed to air it is easy to cause dangerous chemical reactions. Therefore, the pure lithium battery model for anode was not accepted. At the same time, JO Besenhard at the University of Munich discovered the reversible ion exchange properties between graphite and cathode with metal oxide.
Following in 1979 at Oxford University, John Goodenough and Koichi Mizushima built a rechargeable battery that created a 4V current using Lithium Cobalt Oxide (LiCoO 2 ) as an anode and pure lithium as a cathode. LiCoO 2 is a positively charged conductor with high stability, so it is possible to supply lithium ions to generate electricity. This capability has opened up the prospect of using LiCoO 2 as an anode for completely new generations of rechargeable batteries.
In 1977, Samar Basu from the University of Pennsylvania demonstrated the feasibility of making and using electrochemical batteries with lithium and graphite electrodes. Shortly thereafter, the model was officially made by engineers at Bell Laboratories (now AT&T laboratory).

Rachid Yazami, who demonstrated the reversible electrochemical properties of lithium in graphite
In 1980, Rachid Yazami continued to demonstrate the reversible electrochemical properties of lithium in graphite. However, organic substances used as electrolytes in this new generation of batteries are degraded during charging. Therefore, Yazami proposed a sustainable solid organic compound during charging as an electrolyte. Yazami's electrolyte model is still used in generations of Li-ion batteries until now.
By 1983, Michael M. Thackeray, Goodnewa and colleagues have determined that they can use Manganese Spinel minerals to make an anode for Li-ion batteries. This type of mineral has good electrical conductivity, low cost and stable operation. Although there are still disadvantages that are gradually consumed during charging, but can still be overcome by chemical correction measures. Until 2013, Manganese Spinel continues to be used for commercial Li-ion battery generations.

Diagram of operating principle of Li-ion battery
In 1985, Akira Yoshino assembled the first battery model based on all the success factors, using carbonate to help keep lithium ions in an electrode to help LiCoO 2 to be more stable in the air. For this reason, the generation of Li-ion batteries has been completed and is much safer than before.

Is this Li-ion battery familiar to you?
In 1991, Sony Electronics officially commercialized Li-ion batteries under industrial scale. So far, most research activities revolve around improving the performance of Li-ion batteries. Besides providing power for mobile phones, laptops, digital cameras, power tools and medical devices, Li-ion batteries are now also used for electric vehicles. This is the most notable battery generation up to the present time due to its specific energy storage level, simple design, high efficiency, stable flow, low maintenance cost and quite eco-friendly. school
This was followed by the fact that Bellcore officially commercialized Li-ion Polymer batteries in 1994 after the research process. The next step is to pin the appearance of a li-ion battery with cathode using manganese, a lithium-phosphate battery that is continuously improved and perfected by scientists to officially commercialize. Scientists predict the next generation of battery generation based on the advancement of nanotechnology to enhance performance and size and battery life.
- History of formation and development of nuclear energy
- IBM designed battery for electric vehicles running 800km
- This will be the most compact standby charger for mobile devices
- Develop a li-ion battery that alerts itself of gas that is about to explode
- Is smartphone battery just charging once a week?
- Development of edible bio-batteries
- Video: Introducing the world's first flexible battery
- Earth History through images (Part II)
- Astronomers map the history of the Universe
- LG launches Flexible Battery Pack for Smartphone
- History of currency development
- Mac 'explodes' in Japan
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum? Nuclear battery smaller than a coin, phone can be used for 50 years without charging
Nuclear battery smaller than a coin, phone can be used for 50 years without charging  Building a battery that can generate electricity from the atmosphere on Mars
Building a battery that can generate electricity from the atmosphere on Mars  New battery works even when folded or cut in half
New battery works even when folded or cut in half  China designs Mars battery for future exploration
China designs Mars battery for future exploration  Scientists are looking for a series of solutions to replace lithium batteries
Scientists are looking for a series of solutions to replace lithium batteries  Why are lithium-ion batteries easy to catch fire and explode?
Why are lithium-ion batteries easy to catch fire and explode? 