Detection of yeast Lsb2 protein promotes Prion formation
Biochemists at Emory University School of Medicine, USA, have identified the Lsb2 protein in yeast that can promote spontaneous prion formation. This protein is unstable, short-lived, especially sensitive to the pressure on cells like heat.
The Lsb2 protein also showed how cells develop ways to control and regulate the formation of prions.Research in yeast has shown that sometimes prions can help cells adapt to different conditions .
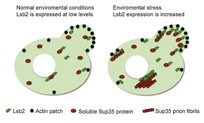
Expression of Lsb2 protein in yeast cells, before the increase in environmental pressure.
Basically prion is a modified protein (abnormal form of protein) rather than a virus, this protein (prion) is present in the bodies of most animals. Prion protein blocks cause mad cow disease in humans and animals. In terms of the mode of transmission mainly due to prions interacting with other proteins in the body, it changes the shape of normal proteins and thereby causes human illness, but they also need some kind of "seed". to begin.
The results of the study are published in Molecular Cell , published July 22, 2011. The authors of the study include: Dr. Keith Wilkinson, professor of biochemistry at Emory University School of Medicine, USA; and Dr. Tatiana Chernova.
In general, proteins are associated with a number of other neurodegenerative diseases such as Alzheimer's disease, Parkinson's and Huntington's disease, in some cases acting like prions. Therefore, the results of the study provided insight into how cells deal with stress, which could lead to the synthesis of toxic proteins that cause many dangerous diseases in humans. .
" It is obvious that Lsb2 protein does not exist in the human body, but there may be a certain protein that performs similar functions ," Wilkinson said. " This pathogenic mechanism can occur in other synthetic proteins, not on the prions available in the human body, in this mechanism: germination and development of modified proteins (abnormal forms). of protein) may play an important role in forming diseases such as Huntington's disease . "
The Lsb2 protein does not seem to be able to form stable prions. The Lsb2 protein is bound to and encourages the collection of other proteins, to form yeast prions Sup35.
"Our research model is to induce a high level of stress for Lsb2 protein, allowing the accumulation of modified proteins (abnormal forms of proteins)," Wilkinson said. "The Lsb2 protein will protect the newly formed prion patterns efficiently ."
This study is funded by the US National Institutes of Health.
- Mad cow disease helps ... increase intelligence?
- For the first time, yeast chromosomes were synthesized
- Latest model of Alzheimer's causes
- Beer will be cheaper and more charming thanks to artificial yeast
- The process of molecular formation determines protein function
- Israel has succeeded in producing beer with yeast from over 5,000 years ago
- Detection of spider silk formation mechanism
- Natural selection promotes species formation
- Beer has yeast extracted from whale fossils 35 million years
- New findings on the risk of infection 'mad cow' in humans
- Successfully tested human gene implants on yeast
- Protein discovery helps to erase painful memories
 Green tea cleans teeth better than mouthwash?
Green tea cleans teeth better than mouthwash? Death kiss: This is why you should not let anyone kiss your baby's lips
Death kiss: This is why you should not let anyone kiss your baby's lips What is salmonellosis?
What is salmonellosis? Caution should be exercised when using aloe vera through eating and drinking
Caution should be exercised when using aloe vera through eating and drinking