Discover new methods to 'Arrest' intermediate compounds in water
A new technique has just been developed by scientists at Lawrence Berkeley National Laboratory of the US Energy Agency, capable of capturing ' intermediate ' compounds that have short but extremely long existence. Weighted, compounds that help bring about chemical reactions take place in aqueous solution from the start of the reaction until the final product is formed.
This new technique basically involves the temporary capture of short-lived and difficult-to-obtain molecules in molecular pyramids.
'Nature is very effective in creating a local chemical environment inside a different protein than the surrounding aqueous solution. This is the method to bond the molecules of the substrate and modify their properties, especially for catalysis, ' said chemist Ken Raymond. 'Now we have proven
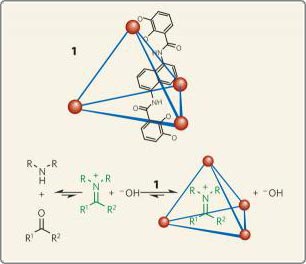
Molecules (1) naturally formed in water from gallium ions (red) and 6 organic molecules (only one of these 6 has a simple structure; their positions are shown by blue lines).Berkeley Lab scientists use these tetrahedra to ' trap ' iminuium ions (Photo: DOE / Lawrence Berkeley National Laboratory)
It is possible that the narrow and modified chemical environment is similar in a water-soluble super-molecular metal complex cluster that can catalyze the molecule of the substrate by making significantly stabilizes reactive intermediates, similar to those of an enzyme. '
Raymond was the one who carried out the study with chemist Robert Bergman. Mr. Raymond is a leading expert in the field of isolated chemical research and Bergman is an expert in chemical reaction mechanisms.
From the initial components to the final component, the process of a chemical reaction usually involves many intermediate steps, especially in aqueous solution. These intermediate steps require many chemical compounds, compounds that can only exist for a very short time and with a very small content.
Although only transiently present, these intermediates are likely to produce a huge impact on the outcome of a reaction . Scientists want to know more about these intermediates, especially because water is a major solvent that nearly all enzymatic reactions and other biological reactions can occur in water. However, the study of unstable high-dose intermediates in water suggests that this is clearly a challenge and chemists are eager to find new ways to separate these compounds. leave other molecules and prevent them from continuing the next reaction.
Bergman said: 'By capturing intermediate ions, we can produce them in higher doses in normal aqueous solutions. When doing this, we can use intermediates in reactions, reactions that may be easily reactive and these side effects will take place when the target compound is present with the function. amount too low. In addition, the ability to create a high level of other intermediates allows us to directly study their basic chemical and physical properties. '
Mr. Raymond and Mr. Bergman, in a study conducted with previous colleagues, Dr. Vy Dong and Barbara Carlm and Dr. Dorothea Fiedler, studied the imin compounds. These are chemical compounds obtained from ammonia and contain a carbon-nitrogen double bond that acts as an important intermediate, in a large number of enzyme-catalyzed biochemical reactions. Interestingly, positively charged ions (cations) of imin compounds will form when amines react with ketone molecules.
'The method to create the type of ion named iminium effectively in a specific reaction mixture requires acid and / or organic solvents,' Raymond said. In aqueous solution, iminium ions only exist very briefly because of their high reactivity to hydrolysis. And because it is fascinated by the ability of host substances, substances that are covalent and can assemble themselves together, in isolating a large number of reactive substances, we sought to Using chemical theory of guest relations to form a novel way to obtain iminium ions (host-guest chemistry - describing mixtures made from two or more molecules or ions bind together in a unique structure by hydrogen bonding, by ion pairs or by van der Waals forces rather than being formed by covalent bonds.

Water solution (Photo: ch.ic.ac.uk)
Earlier, Raymond and his team developed a water-soluble tetrahedron and characterized a water-resistant chamber, which tends to connect, and thus tends to capture cation. . Some of these " trapped " cations will become inert for a prolonged period of time while other cations have unusual reactions in controlling tetrahedral cavities.
Raymond, Bergman and colleagues discovered that the iminium cation-capture tetrahedron naturally formed in water from a mixture of gallium ions, amines and ketones. These tetrahedra immediately captured the iminium cation in their chambers. Based on NMR spectroscopy, anywhere from 30 to 90%, the molecular tetrahedral cavity captures iminium cations, depending on the size of the amino and ketone molecules in the tetrahedron.
'Once captured, iminium ions will become stable for months at normal temperatures,' Bergman said. 'This technique helps us to study the reaction of individual molecules in a controlled and separate environment.'
The technique of capturing molecular tetrahedra developed by two chemists Raymond and Bergman has many characteristics in the same essential characteristics, which are enzyme catalysis , including the creation of quartet cells. molecular interface, allowing recognition and control of asymmetry in reactions. The ability to identify and control such asymmetry is essential in the preparation of bioactive compounds.
The techniques of Raymond and Bergman are also significant for the nanotechnology industry, which is the ability for scientists to organize molecules in molecules and the ability to store information. With this technique, scientists can also develop chemical reactions that were not previously possible in aqueous solution.
Bergman said: 'Our number one priority is to create more types of matter that can be captured by developing the synthesis of nanotubes with wider compartments, to use the opposite properties ( chirality of nanotubes in catalyzing selected reactions, and for capturing metal complexes with active catalytic methods and controlling their selectivity using shapes and the size of the compartment. '
Thanh Van
- New chemical treatment methods make water safer to drink
- What are the dangers of fabric dyeing water?
- Discover compounds that make old books smell good
- The reason dog hair smells bad when it comes to water
- Lose weight but just drink each water? Is it true that life is just pink like that?
- Unknown benefits from water
- How to treat dirty water into clean water
- Russian chemists discover new compounds
- First saw water on meteorites
- Simple recipe for safe drinking water
- Children bullied easily abused alcohol
- Vitamin C and water are not only good for humans but also for plastic.
 'Barefoot engineer' invents a pipeless pump
'Barefoot engineer' invents a pipeless pump Process of handling dead pigs due to disease
Process of handling dead pigs due to disease Radiometer
Radiometer Warp Engine: Technology brings us closer to the speed of light
Warp Engine: Technology brings us closer to the speed of light