Revealing the strange properties of glass
Scientists had a groundbreaking discovery of the strange properties of glass expression in both solid and liquid form.
This discovery is a step towards the same kind of aircraft as Wonder Woman (a role in Justice League). This aircraft can have wings made of glass or metal glass, not completely invisible.
This breakthrough includes solving the problem of the nature of glass that has been around for decades. Despite the solid shape, glass and gel are in fact "stuck" - the intermediate form of liquid and solid - and move very slowly. Like cars with traffic jams, the atoms inside the glass cannot reach where they want because the pathways are blocked by the atoms nearby. So according to chemists and materials scientists, although glass is considered solid, it has never really become a complete solid.
So far, new studies have focused on understanding this congestion, but Paddy Royall of Bristol University and colleagues in Canberra and Tokyo have shown that glass cannot become solid because of the original structure. The special element forms glass when it cools.
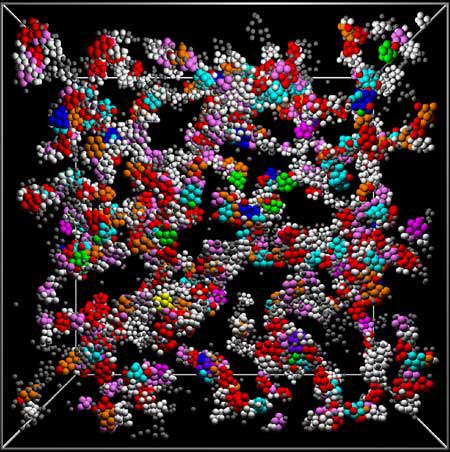
The glue element, like an atom, forms a solid substance in the structure of glass.(Photo by Paddy Royall, Bristol, UK)
Icosahedron stuck
Royall said some crystallized materials when they cooled, arranged atoms in a well-proportioned system called the trellis. Although the glass 'wants' to become crystal, when it cools the atoms blocked in an almost random arrangement, it prevents it from forming a normal trellis.
In the 1950s, Sir Charles Frank of the Department of Physics at Bristol argued that this arrangement constituted an icosahedron (twenty-face block), but at that time he was unable to prove his theory.
Royall explained that an icosahedron is a three-dimensional pentagon, you cannot slice the floor with pentagons, you cannot fill three-dimensional space with icosahedrons. So you cannot form a trellis from pentagon blocks.
In Frank's opinion, there is always a competition between crystal formation and pentagons in glass, which prevents crystal formation. If you cool a liquid and it creates a lot of pentagons, the pentagons will exist and the crystal cannot form.
Royall said Frank was right, and his team proved this through experimentation. You cannot see what happens to atoms when they cool because they are so small, so Royall and colleagues used special molecules called atomic colloids, but big enough to can be viewed with a specialized microscope. The team cooled some and observed developments.
They found that the gel that these molecules formed also "wanted" to become crystals, but could not because of the formation of icosahedra-like structures - just as Frank had predicted.
Royall said: 'The formation of these structures is the basis for' stuck 'materials while explaining glass as glass and not a liquid - or solid'.
These findings are described in detail in the June 22 issue of Nature Materials. The study was partly funded by the Ministry of Education, Culture, Sports, Science and Technology and the Royal Council.
Prevent aircraft disaster
Understanding the structure of atoms when cold glass is an important breakthrough in knowledge of durable materials and at the same time opens the era of light and durable materials called metallic glass. - has been used to make some types of golf clubs. This material is glossy black, not transparent, because it has a lot of free electrons (like mercury in old-fashion thermometers).
The metal often crystallizes when it cools, but the pressure accumulated along the boundary between the crystals can lead to a breakdown of the metal structure.
For example, the world's first jet aircraft, De Havilland Comet, fell for this reason. If the metal is made to cool when it has a glass-like structure and the crystals do not form a boundary, it will be harder to break. , In addition to golf clubs, metal glass can be suitable for many types of products that need flexibility such as airplane wings or machine parts.
The nature of glass differs from its shape
Royall is one of the scientists who claims that if you wait long enough, maybe billions of years, all glass will eventually crystallize into a real solid. In other words, glass is not in equilibrium (although it seems so in our limited life).
Royall told LiveScience: 'This has not been widely recognized. Our job is to try to make this argument more widely accepted. I think there is growing evidence that glass' wants' to be crystal '.
Royall continued: 'However, glass looks like liquid, this is also a mystery that we have spent a lot of research on. Many believe that glass has a fluid-like structure and so it looks like liquid. But in fact it has no structure of a liquid '.
- The bullet shattered when it shot into a glass drop.
- New type of shockproof glass inspired by nature
- Glass has the ability to 'transform' when meeting water
- Nanotechnology has been around since the 4th century?
- Moth eyes suggest researchers to develop self-glazing, anti-glare glass doors
- What is glass?
- Unexpected uses of Gorilla Glass
- Smart glass
- 'Glass rain' falls on Hiroshima beach
- How are the strengths produced?
- Discover 1,600-year-old glass furnace in Israel
- Car glass smashes in an emergency situation is more difficult than you think
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum? Pharaoh Tutankhamun possessed treasures from outside the Earth?
Pharaoh Tutankhamun possessed treasures from outside the Earth?  Chilling revelations from 1,800-year-old glass 'treasure' in Antarctica
Chilling revelations from 1,800-year-old glass 'treasure' in Antarctica  The core of a shattered planet fell on Australia?
The core of a shattered planet fell on Australia?  Invention of the most durable material ever
Invention of the most durable material ever  Invention of glass that can generate electric current
Invention of glass that can generate electric current  Man has the ability to balance objects, defying the laws of physics
Man has the ability to balance objects, defying the laws of physics 