This new material will help us light up the house without using electricity
Room-temperature phosphorescence is when a material absorbs short-wavelength energy (such as UV light) and then emits it as visible light.
This is in contrast to a fluorescent material, as it will immediately return to light and cease to glow when the light is turned off.
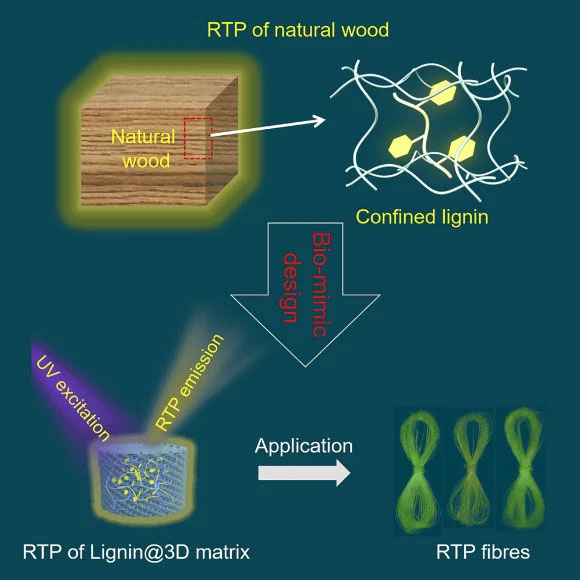
Professor Zhijun Chen, a researcher at the Advanced Wood Materials Engineering Research Center at Northeastern Forestry University, and colleagues found that natural mahogany and weak phosphorus release light light for a few milliseconds because lignin is trapped in the 3D matrix of cellulose.
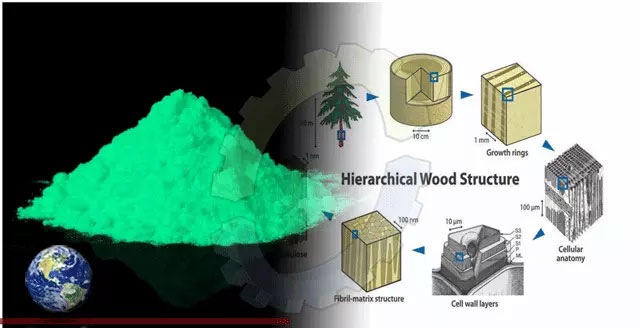
Consumer demand for renewable, environmentally friendly materials has prompted scientists to research wood-based thin films for optical applications. To date, however, these materials have often had disadvantages, such as poor mechanical properties, uneven sheen, lack of water resistance, or the need to cover with petroleum-based polymer resins.
This inspired them to mimic the luminous properties by cross-linking the lignin in the 3D polymer network, causing it to glow more clearly for longer periods of time.
"It was really an unexpected and exciting discovery," said Professor Chen.
"We think this work will not only provide a new option for sustainable light-emitting materials, but also a new route to fully utilize the values of lignin."
The researchers discovered that by varying the cavity sizes in the 3D polymer network and the polymer drying times, they could change the lifetime of phosphorus.
Phosphorus, or luminescence, is a form of luminescence in which molecules of phosphor absorb light, converting the energy of photons into the energy of electrons in some quantum state with energy levels. high but stable in the molecule so that the electron then slowly drops to a quantum state at a lower energy, and releases some of the energy back in the form of photons. Phosphorus differs from fluorescence in that the return of electrons to their former state, accompanied by the release of photons, is very slow. In fluorescence, the electron's fall back is almost instantaneous; causing the photon to be released immediately. In that gas, for phosphorescence, it acts as light stores: capturing light and then slowly releasing it.
"All lignin is weakly luminous, but most of the light energy is lost due to vibrations or movement of the lignin molecules, meaning it cannot be seen clearly with the naked eye," said Professor Tony James. , said a researcher at the Center for Sustainable Circular Technology at the University of Bath.
"We've found that immobilizing lignin in an acrylic polymer causes more energy to be released as light - in other words, the less it vibrates, the more it glows."
"Most current luminescent materials carry toxic components or are difficult to prepare, so we wanted to develop a new material that overcomes these limitations."
"Although this research is still new and has not really explored all aspects, our new material shows great potential in creating a non-toxic, biodegradable phosphorescent material. biological, more stable, can be used in a wide range of applications".

Phosphorous substances are applied to create light sources for situations where there is temporary lack of light but do not need to consume energy to feed. The luminous energy has been stored since the substance was exposed to natural light. For example, they are mounted on the dial of a wristwatch, helping to read the time in the dark; mounted on a compass pointer, to determine direction in the dark; or mounted on an electric light switch, indicating the position of the light switch when the light is not on. They are also used to make decorations, making light-emitting inks (although fluorescent inks are more common). Laser fabrication can also use phosphors.
The reason is that electrons can stay on the excited state long enough to wait for other photons to pass through and cause co-phase stimulated emission. Phosphorus have also been used in cathode ray screens. After the flow of electrons hits a pixel of the screen, the spot, which contains phosphors, is excited and continues to glow a short time later. However, fluorescent materials can also be used, thanks to the retention effect on the retina. Similar to a cathode ray screen, a screen that records high-energy particles (electrons, X-rays, neutrons, etc.) may also contain phosphors; although fluorescent agents may also be used.

The origin of the name "phosphor" is due to the light emitted in the dark by phosphorescence-like phenomena, emitted by compounds of phosphorus in an oxidizing chemical reaction in air.
The name has been used to describe substances that glow in the dark without burning, since the German alchemist Hennig Brand discovered phosphorus in 1669 through the preparation of urine.
He noticed that the substance he had just prepared glowed in the dark. The word phosphor itself is derived from the Greek word phosphoros, meaning "light carrier". However, the physical nature of the two phenomena is different; in which the light of a phosphor derives its energy from a chemical reaction. The phosphor glow that Brand saw was actually caused by the phosphor burning smolderingly and slowly in air.
- The house itself produces electricity and changes color
- Solar homes can resist storms
- The house is made of 3D wood in the future
- New quantum matter can conduct electricity at nearly the same speed as light
- New invisible material created by light
- The darkest material can swallow most of the light
- Mexican artists create a rainbow in the house
- Create a super-elastic material that can generate electricity when pulled or compressed
- The new form of material resembles a fictional sword of light
- The Japanese company created a fabric that could 'shock' the bacteria
- Implementing a project to produce electricity by light engine
- Turn the light on and off with spin
 Vietnam 5th Asian champion on fuel-efficient vehicles
Vietnam 5th Asian champion on fuel-efficient vehicles We can read all NASA studies completely free of charge
We can read all NASA studies completely free of charge Singer and songwriter Bob Dylan won the 2016 Nobel Prize for Literature
Singer and songwriter Bob Dylan won the 2016 Nobel Prize for Literature Scientific revolution in Asia
Scientific revolution in Asia