For the first time, a nanomaterial has been created that is both liquid and solid
For the first time in the world, researchers have synthesized a type of nanomaterials both in solid and liquid form at temperatures from -93 ° C to 530 ° C.
If we drop an ice cube in a glass of water or another liquid, we know that the stone will melt at room temperature. And if the glass is placed in the freezer, the water in the glass will freeze around the stone. According to the laws of physics, if you want to keep all in solid state, you must maintain the correct temperature at 0 o C.
The material used here is gallium.
But for the first time in the world, researchers have just synthesized a type of nanomaterial that is both liquid and solid in temperature ranges from -93 ° C to 530 ° C. This groundbreaking research could pave the way for the production of new devices in the field of electronics and chemistry.
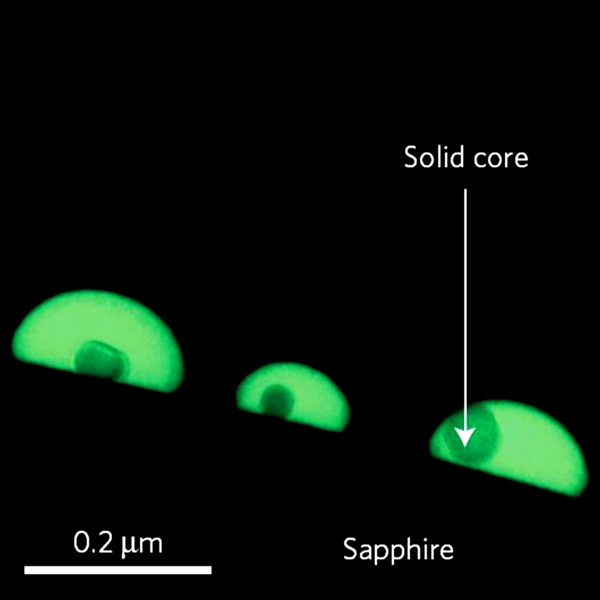
The material used here is gallium . This metal (Ga symbol) is usually solid when the temperature is below 29.76 ° C and above this temperature will liquefy. This is a simple molecule made up of galli atoms and widely used in solid form in the electronics industry.
Accordingly, the researchers created gallium droplets about 50 nanometers in diameter (one billionth of a meter) and placed them on the sapphire crystal (or aluminum oxide crystal). As a result, the liquid gallium rushed towards the center and turned into a solid form in the form of a crystal. And what's interesting is that the gallium around is still . liquid, similar to the drops of water surrounding a stone. Thus, this gallium drops is both solid and liquid in a temperature range of 620 ° C (from -93 ° C to 527 ° C).
This phenomenon is completely against the previous physical laws of solids and liquids, never known until now, even theoretically. Therefore, this is an infinite potential in nanotechnology with the electronics, IC, chemical industry (catalysts and molecular sensors), ultraviolet sensors .
- Solids can be harder due to the liquid
- Glass is liquid at normal temperature?
- The culprit turns solid objects into liquid, sinking cargo ships
- Scientists create a new physical state - both solid and liquid
- The liquid boils and freezes at the same time
- This semi-solid, half-liquid material can 'heal' itself
- For the first time, humans created such
- The solid-state battery replaces the Li-ion battery with an important step forward for applications to smartphones
- Super-materials transform instantly from solid to liquid
- Video: Liquid solidified immediately when punched
- Artworks made of liquid nitrogen
- The dish containing liquid nitrogen punctured the baby's stomach
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum? The secret behind China's 'explosive carving' craft
The secret behind China's 'explosive carving' craft  Earth has 'matter in another dimension', scientists have been confused for 90 years
Earth has 'matter in another dimension', scientists have been confused for 90 years  Solid-state batteries will replace Lithium-ion batteries
Solid-state batteries will replace Lithium-ion batteries  The solid-state battery replaces the Li-ion battery with an important step forward for applications to smartphones
The solid-state battery replaces the Li-ion battery with an important step forward for applications to smartphones  Ants change shape when fighting
Ants change shape when fighting 