Scientists create unprecedented isotope of magnesium
The world's lightest magnesium isotope was created, giving new insights into the binding energies of electrons as they orbit the nucleus.
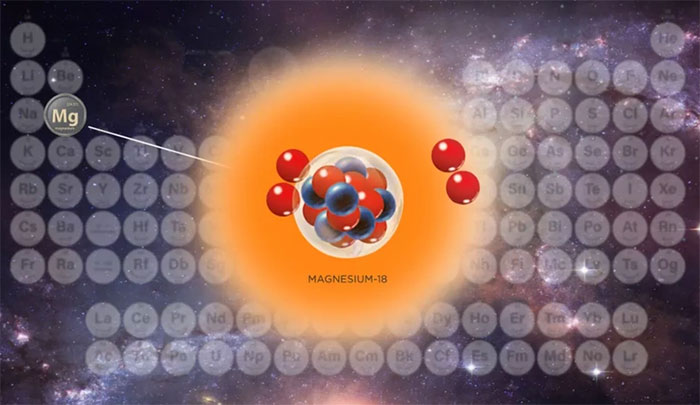
This isotope is called magnesium-18.
Under normal conditions, pure magnesium is a soft, gray metal with the atomic number 12. This indicates that it has 12 protons - or positively charged particles - in its nucleus.
But recently, scientists have created the world's lightest magnesium isotope, with only 6 neutrons in its atomic nucleus. This isotope is called magnesium-18.
Although it decays too quickly to be directly measured, the researchers hope their discovery will help better understand how atoms are made.
Thanks to such exotic isotopes, there may be more versions of a chemical element, with more or less neutrons in the nucleus than usual. Reportedly, this helps define the limits of the models scientists use to figure out how atoms behave.
According to the team, the radioactive decay product of the isotope provides new insights into the binding energies of electrons orbiting the nucleus. "We're measuring things we can measure to predict things we can't," said Kyle Brown, a chemist in the Rare Isotope Rays Research Facility at Michigan State University.
To date, scientists have identified several thousand isotopes of 118 common elements in the periodic table, and new isotopes are discovered every year.
- How to use Magnesium B6 for safe treatment
- Magnesium is more important to children's bones than calcium
- Diet high in magnesium prevents colon cancer
- What is carbon isotope analysis? Why is it possible to date the archeology?
- Unexpected causes cause insomnia, many people suffer without knowing
- 10 types of magnesium rich foods are extremely good for health
- Detecting cells with age equal to the age of the body
- Use magnesium to reduce vehicle weight and save fuel
- How to detect osteoporosis at the earliest stage
- Detection of Fukushima radiation on the Canadian coast
- What is heavy water?
- Radioactive substances appear in Europe
 'Fine laughs' - Scary and painful torture in ancient times
'Fine laughs' - Scary and painful torture in ancient times The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why?
The sequence of numbers 142857 of the Egyptian pyramids is known as the strangest number in the world - Why? History of the iron
History of the iron What is alum?
What is alum?