When lacking one of the two enzymes, the nematode keeps a low-calorie diet that will have a different lifespan than normal worms. This is the result reported in the June 24 online edition of Nature.
'In addition to the two enzymes mentioned above, the only remaining factor also adjusts the life span corresponding to the diet at the end of the signal layer,' said Dr. Andrew Dillin, lecturer of the Molecular Biology Laboratory. and Cell, author of the study. 'These two enzymes are at the top of the signal layer, allowing us to get closer to the receptor that perceives the signal to promote a healthier life.'
The identification of this receptor may allow researchers to create counterfeit drugs as signals and therapies for age-related diseases. This allows us to gain the benefits of eating less without necessarily adhering to a complete diet.
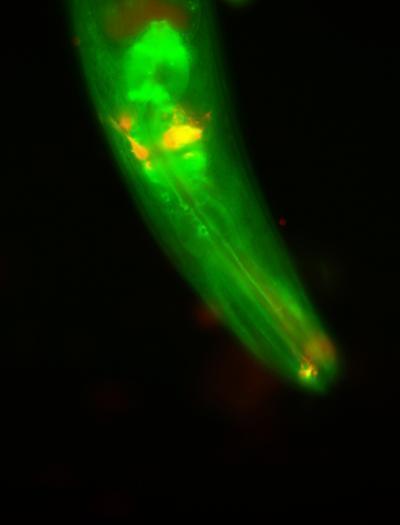 Enzymatic WWP-1, shown in blue, is an important element in the signaling link between limiting food intake and prolonging life in the nematode. Sensory neurons are shown in red. (Photo: Dr. Andrea C. Carrano, copyright of Salt Biology Research Institute) Although lifestyle-related factors such as obesity have a clear effect on life expectancy, genetic factors It is also thought to play a central role in the aging process. To date, only three genome systems can work to ensure the youthfulness of the body. A genome lies on insulin-1 growth factor that regulates the body's metabolism and growth; the second genome is regulated by mitochondria, and the third genome is associated with a low diet.
Enzymatic WWP-1, shown in blue, is an important element in the signaling link between limiting food intake and prolonging life in the nematode. Sensory neurons are shown in red. (Photo: Dr. Andrea C. Carrano, copyright of Salt Biology Research Institute) Although lifestyle-related factors such as obesity have a clear effect on life expectancy, genetic factors It is also thought to play a central role in the aging process. To date, only three genome systems can work to ensure the youthfulness of the body. A genome lies on insulin-1 growth factor that regulates the body's metabolism and growth; the second genome is regulated by mitochondria, and the third genome is associated with a low diet. But author Andrea C. Carrano, PhD, who works at the American Cancer Society, did not intend to elucidate the molecular relationship between limiting feeding and prolonging life expectancy when she began investigating the role. Game of WWP-1 enzyme. ' I just knew then that WWP-1 is an ubiquitin ligase, and that mammalian cells contain up to three copies - which makes it difficult to study WWP-1's ability.'
Because the roundworm Caenorhabditis elegans only contains a single copy, Carrano teamed up with Dillin of the Salt Institute, who studies aging and longevity in Caenorhabditis elegans. Early experiments revealed that worms that do not have WWP-1 genes seem to have nothing unusual except for stress. "This finding is the first sign that WWP-1 may play a role in aging because changes that affect stress are often associated with life expectancy," she said.
After these findings, Carrano's next series focused on WWP-1's potential role in regulating life expectancy. When she conducted gene interference on the worm to clarify the role of WWP-1, the worms fed in a reasonable manner increased an average of 20% of life expectancy. The elimination of PHA-4, the gene discovered by Dillin's lab, the only gene to date is recognized as having a decisive role in prolonging the life-span corresponding to reduced diet, causing long-term loss of efficacy. Lifespan of WWP-1 implant extra. When there is no WWP-1, reducing calorie intake does not have an effect on longevity.
When a study conducted by other scientists found that the UBC-18 interacts with WWP-1, Carrano wonders if it plays any other role in prolonging life through reduced diets. is not. She first confirmed that the UBC-18 acts as an enzyme paired with ubiquitin (a common form of protein in cells - ND) and has a supportive effect for WWP-1. then she checked to see if it had a role in adjusting life expectancy. 'Overexpression of the UBC-18 is not enough to extend the life of the worm, but eliminating it will undermine the effect of reducing food intake,' Carrano said.
'WWP-1 is quite similar in worms and humans, and it can play a role in the aging process in humans,' said Dr. Tony Hunter, senior author, lecturer of the International Biological Laboratory. Cells and Molecules, said. 'We didn't think this protein would involve regulating lifespan, but interesting experimental results led us to a new direction of surprise.'
This work is supported by the National Institutes of Health, Ellison Medical Foundation, Glenn Foundation and the American Cancer Society.
Dr. Zheng Liu, Ph.D., a research collaborator working at Dillin Laboratory also contributed to this scientific work.
 Green tea cleans teeth better than mouthwash?
Green tea cleans teeth better than mouthwash? Death kiss: This is why you should not let anyone kiss your baby's lips
Death kiss: This is why you should not let anyone kiss your baby's lips What is salmonellosis?
What is salmonellosis? Caution should be exercised when using aloe vera through eating and drinking
Caution should be exercised when using aloe vera through eating and drinking