Decode the ability to recognize the 'super-accurate' old person of the immune system
For us, it is not easy to recall someone's face 30 years ago. However, with small cells in the immune system, remembering the 'enemy' has become instinctive.
Many questions regarding this memory storage capacity have been raised for years, but there is still no satisfactory answer.
A recent study has completed the process of remembering the body's pathogens. Since then, the solution to the ability to remember immune cell information as a synthetic library of past 'battles' has also been revealed.
Superhuman memory ability of "veterans"
Scientists from the University of California, Berkeley used hydrogen isotopes to mark the inside of volunteers. From there, they followed a specially selected virus from the beginning of the infection to the completion of the mission.
In fact, the work of perfecting the whole immune process and the ability to remember and eliminate particular pathogens has been underway for several centuries before. Studies show that our bodies have many different types of white blood cells that are capable of detecting and destroying invasive cells. Two of them are B cells, which help form and secrete antibodies that act as '1 name tags' attached to harmful cells; and T cells, perform a variety of immune-related tasks such as identifying harmful particles from the outside.
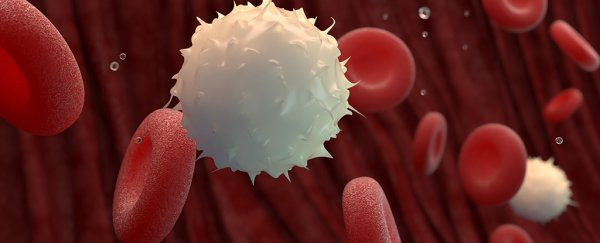
White blood cells.
These two types of cells act together as 'historians', noting the remnants of the battle as veterans. This is exactly how the immune cells carry out the task of detecting and remembering the events that took place - at least in terms of chemistry - and storing them for a long time.
The project nutritionist and lead author, Marc Hellerstein from Berkeley University, said: ' This work helps solve the fundamental questions about the origin and life of human memory cells CD8 + T. , which was created after acute infections'.
Dubbed a 'killer cell', CD8 + T toxic cells are produced inside the thymus to identify 'familiar' cells. After ensuring that the body is not infected with old cells, they are freed to 'hunt' more dangerous objects such as cancer cells, bacteria or virus-infected cells.
When the target is identified, the body stimulates the production of this extra T-cell. From there, a small 'army' will release chemicals and spray on 'enemy' cells, creating holes in the surface membrane structure and destroying them.
Not all poisonous T cells are fighting to kill the dangerous cells. Some will play a precautionary role to promptly prevent the attack of old tumors or pathogens if they come back.
Ability to manufacture new vaccines
To better understand this process, the researchers gave 40 volunteers to drink deuterium-containing water (heavy hydrogen) instead of the standard hydrogen to mark all new cells their bodies created at intervals. different time. Next, researchers will prevent yellow fever with live virus that has been weakened - this is also the virus that is not where the volunteers live.
With the formation of new CD8 + T cells, researchers can track these cells continuously for months to understand their numbers and chemical changes. At the same time, there was an interesting finding. When the body begins to respond to the vaccine dose, a series of memory cells are created. Cells that look like T-toxic cells at a young age, only with a single difference, their genes are marked with signs of genetic genetics with a type of cell that was previously destroyed. kill.
'These cells are like veterans. They camped inside the blood and tissues, where the previous battles took place and were just waiting for the appearance of yellow fever ' - Hellerstein said. 'They will rest in silence and disguise as new soldiers. However, they are really experienced people, ready to embark on action and counterattack strongly if the invaders come back. '
This quiet time is the secret of the success of these cells. This is the time they quietly hide themselves and are ready to move into a state of fullness, to act in time when the pathogens return.
On average, a T cell will have a half-life of 30 days. That means that after about a month, white blood cells almost die. These camouflaged T cells have a half-life of up to 450 days. As a result, they can stick continuously for years, if not for decades. The growing understanding of the memory system of immune cells helps us know how to exploit in a way that benefits people.
'Understanding the basis of long-term benefits thanks to the immune memory system will help scientists make better vaccines, better understand the differences between diseases and diagnoses. Hellerstein is optimistic about the future of this study.
- Close-up of the human body's immune system is beautiful
- Video: Dance of the beautiful light of the drone
- Fasting 8 days a year helps to maintain the immune system
- Foods that are harmful to the immune system
- After watching this video, you will see how amazing our immune system is
- Why can't the body resist HIV?
- The boy was born without an immune system
- The immune system becomes the same when we are together
- Crow can recognize human voices
- Tumors damage the immune system
- Genetic contact between the immune system and the nervous system
- New findings: Depression may be due to an overactive immune system
 Green tea cleans teeth better than mouthwash?
Green tea cleans teeth better than mouthwash? Death kiss: This is why you should not let anyone kiss your baby's lips
Death kiss: This is why you should not let anyone kiss your baby's lips What is salmonellosis?
What is salmonellosis? Caution should be exercised when using aloe vera through eating and drinking
Caution should be exercised when using aloe vera through eating and drinking Good news: New cancer drug discovered that causes cancer cells to 'starve'
Good news: New cancer drug discovered that causes cancer cells to 'starve'  Why do we get older when cells are always replicating?
Why do we get older when cells are always replicating?  Shocking Research: Human Memory Doesn't Exist Only in the Brain
Shocking Research: Human Memory Doesn't Exist Only in the Brain  Reasons for easy weight gain: Obesity has 'memory' and retains mechanisms in cells
Reasons for easy weight gain: Obesity has 'memory' and retains mechanisms in cells  What happens to cancer cells after they die?
What happens to cancer cells after they die?  New Breakthrough: 'Hack' Tumors, Turn Cancer Cells Into Pills
New Breakthrough: 'Hack' Tumors, Turn Cancer Cells Into Pills 